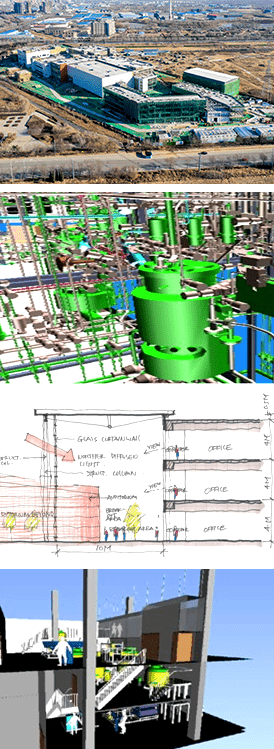
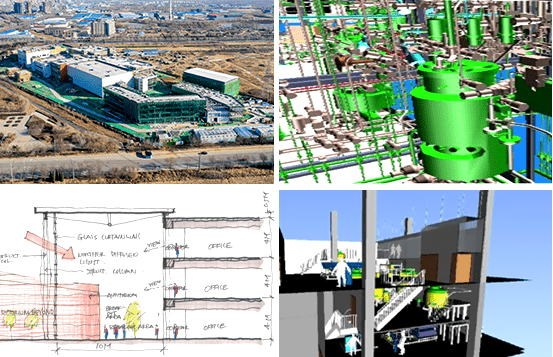
EPI Engineering founded in 2006 as an employee owned consulting company. We are now an engineering firm and providing professional facility engineering services in pharmaceutical and biotechnology industries for many years.
In collaborating with selected local architectural design institutes we are expanding business to provide our design of excellence services in North America and emerging countries. We facilitate single point to work with our project owners whilst ensure the modern GMP biopharmaceutical manufacturing facility design incorporates local codes and field constructability.
The core competence of EPI is related to its Subject Mater Experts (SME). They are highly qualified senior engineers whose professional abilities derived from profound theoretical knowledge and long years experiences globally.
EPI has delivered over twenty projects successfully in the past ten years. Our main track records are from pilot scale single 200L cell culture plant throughout multiple 5kL commercial manufacturing GMP facility. Our execution model allows EPCMCQv one-stop-shopping service to be provided.
The process platforms of biopharmaceutical plants that we designed includes,
CHO, BHK, and Per.C6 mammalian cell culture express monoclonal antibody, fusion protein, human vaccine, animal vaccine, halmon etc.
E.coli and yeast intracellular systems based VLP vaccine, HSA, insulin etc.
T-cell based micro-manufacturing plant with benchtop separation and cell culture systems. It provides 50ml scale operations to enable immunological therapeutic materials preparation as well known the CART.
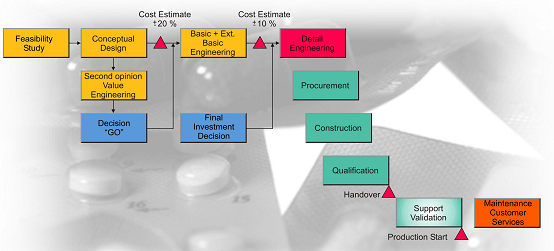
During engineering delivery, we help project owner to analyze manufacturing process structure using specialized modeling tools, ensuring your facility to be more multipurpose to products, and smarter production on systems to save capital investment. The industrial 4.0 methodologies are also embedded. The design focuses on LEAN manufacturing operations, automated internal logistic, artificial intelligence on golden batch study, continuous manufacturing.
▸ Process Specialists ▸ Architects ▸ Engineers